

Catalog Number: I001208
Strain Name: C57BL/6NCya-Dmdtm3(mDmd Exon 44-45 del; hDMD Exon 45 ins)/Cya
Genetic Background: C57BL/6NCya
One of Cyagen's HUGO-GT™ (Humanized Genomic Ortholog for Gene Therapy) Strains
Strain 설명
Duchenne Muscular Dystrophy (DMD) is a severe, progressive, and disabling X-linked recessive genetic disorder characterized primarily by muscle atrophy. This disease leads to motor impairments, eventually requiring assisted ventilation, and often results in premature death. The primary cause of DMD is mutations in the DMD gene, which encodes the dystrophin protein. These mutations lead to a reduction or absence of dystrophin in muscle tissue, resulting in muscle atrophy and related complications [1]. The lack of dystrophin leads to the breakdown of the dystrophin-associated protein complex (DAPC) within the muscle membrane, disrupting the interaction between actin and the extracellular matrix, making the muscles more susceptible to damage. This susceptibility results in the gradual loss of muscle tissue and function, potentially leading to cardiomyopathy [2]. Researchers have identified thousands of different DMD gene mutations in patients with DMD. Deletion mutations account for approximately 60%–70%, while duplication mutations account for 5%–15%. These mutations are primarily concentrated in hotspot regions of the DMD gene, specifically between exons 45-55 (47%) and exons 3-9 (7%) [1].
Currently, gene therapy approaches for Duchenne Muscular Dystrophy (DMD) primarily include exon skipping and AAV supplementation, as well as emerging gene editing techniques like CRISPR. The exon skipping strategy involves using antisense oligonucleotide (ASO) drugs to bind to specific sequences of pre-mRNA, skipping the mutated exon and restoring the open reading frame (ORF) integrity, thus producing a truncated but partially functional dystrophin protein. Several ASO drugs targeting the DMD gene have been approved, such as Eteplirsen (targeting exon 51), Golodirsen (targeting exon 53), and Casimersen (targeting exon 45) developed by Sarepta, and Viltolarsen (targeting exon 53) developed by Nippon Shinyaku. Since most ASO and CRISPR-based gene editing therapies target the human DMD gene, humanizing mouse genes helps accelerate clinical applications for DMD therapies, considering the genetic differences between animals and humans.
The B6-hDMD(E44-45)*Del E44 mouse is a humanized model of the Dmd gene, where exons 44-45 and the flanking regions of the mouse Dmd gene are replaced with the corresponding sequences of exon 45 and its flanking regions from the human DMD gene (i.e., exon 44 of the human DMD gene is deleted). This model is suitable for researching Duchenne Muscular Dystrophy. In addition, based on the independently developed TurboKnockout fusion BAC recombination technology, Cyagen provides other humanized models such as [hE49-53, del E50], [hE49-53], [hE44-45, c.6438+2 T to A], [hE8-30], covering most popular research areas and offering customized services based on different mutation needs.
Strain 유전자 편집 전략
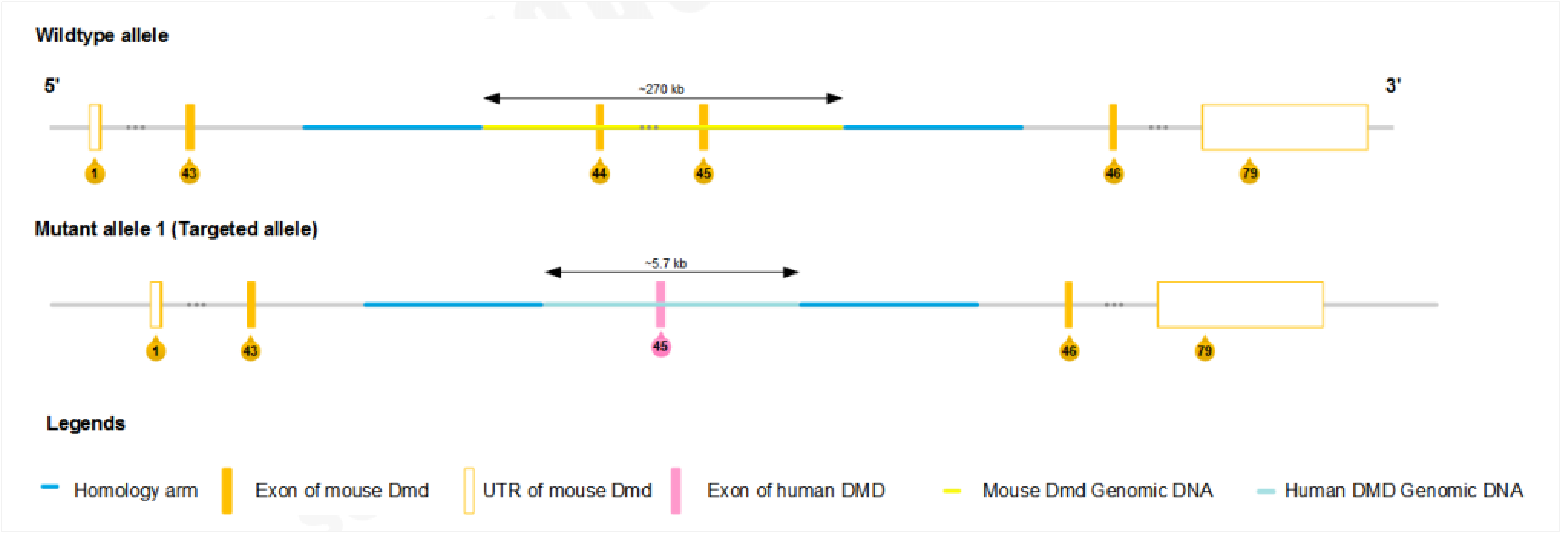
Figure 1. Gene editing strategy of B6-hDMD(E44-45)*Del E44 mice. The sequences from ~2.5 kb upstream of exon 44 to ~2.9 kb downstream of exon 45 of mouse Dmd were replaced with human DMD exon 45 plus flanking region (~2.5 kb upstream of exon 45 to ~3 kb downstream of exon 45) cassette.
응용 분야
검증 데이터
1. RT-PCR
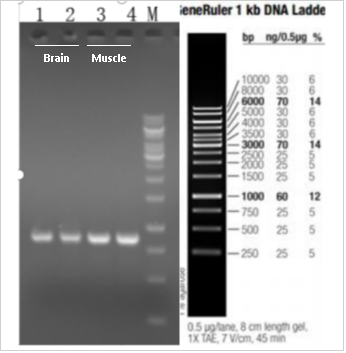
Figure 2. RT-PCR detection results of gene expression in B6-hDMD(E44-45)*Del E44 mice. Using primers specifically paired with human DMD mRNA, reverse transcription was performed to obtain complementary DNA (cDNA), followed by gel electrophoresis to determine the expression of the human DMD gene. The result shows the presence of human DMD cDNA bands in both the brain and muscle tissues of B6-hDMD(E44-45)*Del E44 mice, with band sizes matching the expected values and no abnormal transcription bands observed.
2. cDNA sequencing
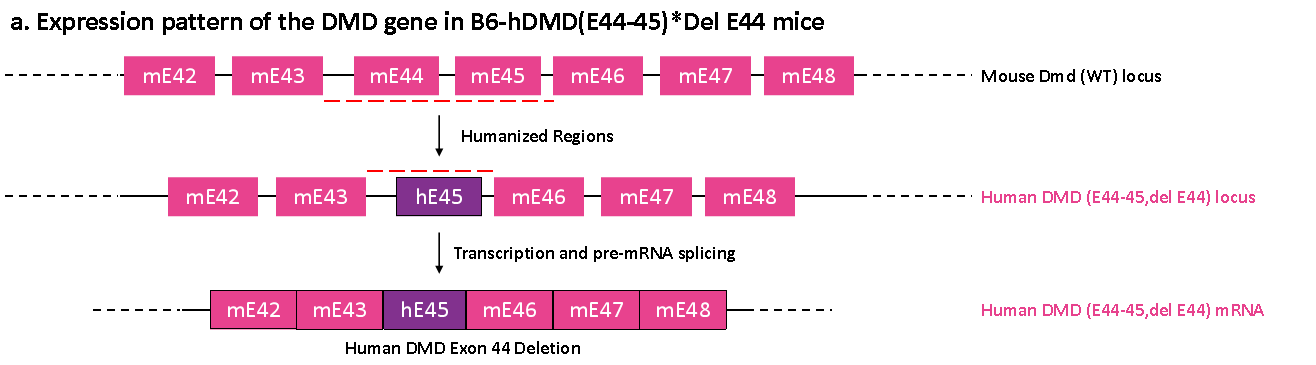
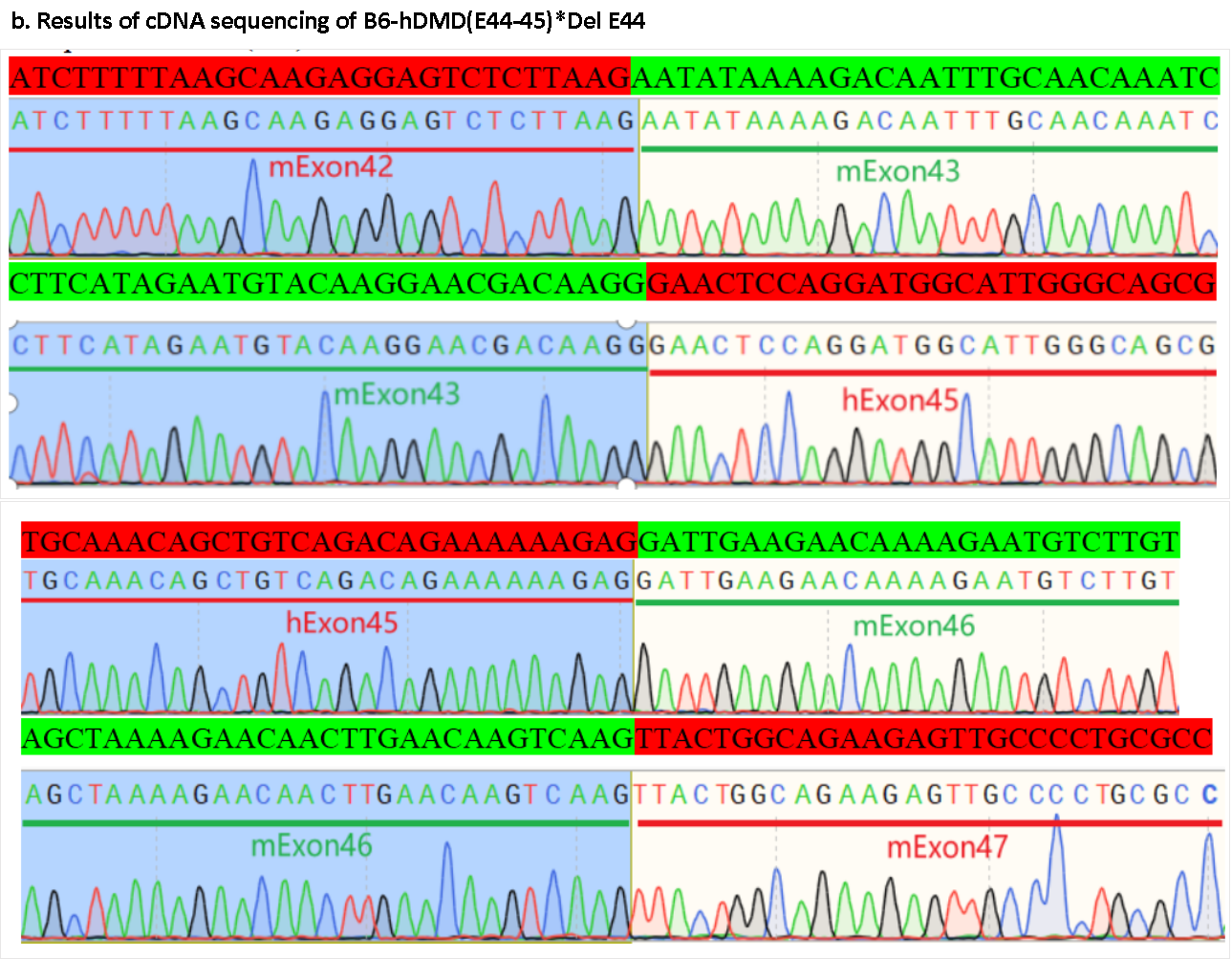
Figure 3. Gene sequencing results of the exon 44-45 region of the Dmd gene in B6-hDMD(E44-45)*Del E44 mice. Sequencing analysis of DMD cDNA obtained from mRNA in the RT-PCR mentioned above test indicates that exons 44-45 of the mouse Dmd gene in B6-hDMD(E44-45)*Del E44 mice have been successfully replaced with exon 45 of the human DMD gene. The sequence matches the reference sequence for exon 45 of the human DMD gene, with no expression of human DMD exon 44 detected.
참고 문헌