

Catalog Number: I001218
Strain Name: C57BL/6JCya-Hctm1(hC5)/Cya
Genetic Background: C57BL/6JCya
One of Cyagen's HUGO-GT™ (Humanized Genomic Ortholog for Gene Therapy) Strains
Strain 설명
The C5 gene encodes a key component of the complement system, primarily produced by hepatocytes in the liver, with macrophages potentially serving as local sources of C5a. As part of the innate immune system, the complement system is activated upon tissue injury or pathogen invasion, playing a crucial role in inflammation, host homeostasis, and defense against pathogens. Complement activation occurs via three main pathways: the classical pathway, the alternative pathway, and the lectin pathway. All three pathways converge to form C3 convertase, which cleaves C3 into C3a and C3b. In addition to promoting opsonization on pathogen surfaces, C3b is also an integral part of C5 convertase (C4b2aC3b or C3bBbC3b). C5 convertase cleaves the C5 precursor protein to produce C5a and C5b. C5a is a potent inflammatory mediator, while C5b initiates the assembly of the membrane attack complex (MAC/C5b-9), which mediates phagocytosis, cell lysis, inflammatory response, immune regulation, and clearance of immune complexes [1-2]. Additionally, in cases of inherited C3 deficiency, thrombin can substitute for C3-dependent C5 convertase, indicating an alternative complement activation mechanism linked to the coagulation pathway [3].
While the complement system is essential for pathogen elimination and maintaining host homeostasis, its excessive activation can lead to tissue damage and uncontrolled inflammation. Imbalances in complement regulatory proteins are associated with complement-mediated diseases, including age-related macular degeneration (AMD), atypical hemolytic uremic syndrome (aHUS), myasthenia gravis (MG), C3 glomerulopathy, and paroxysmal nocturnal hemoglobinuria. C5 has been identified as a promising therapeutic target in complement-mediated diseases, as inhibiting C5 can block the production of the highly pro-inflammatory C5a and MAC, while preserving the opsonizing functions of C3b and C4b and the immune signaling mediated by C3a [4]. Currently, approved C5 inhibitors are predominantly monoclonal antibodies, such as eculizumab, vilobelimab, and crovalimab. The development of a mouse model expressing human C5 is crucial for the preclinical evaluation of the pharmacodynamics and pharmacokinetics of C5 inhibitors.
The B6-hC5 mouse model is a humanized model of the Hc gene, with the mouse Hc gene homologous to the human C5 gene. Using gene-editing technology, the mouse Hc gene was replaced with the human C5 gene while retaining the mouse signal peptide; the humanized region also includes the 3’ UTR. In addition, based on the independently developed TurboKnockout fusion BAC recombination technology, Cyagen can also generate mutation models based on this strain and provide customized services.
Strain 유전자 편집 전략

Figure 1. Gene editing strategy of B6-hC5 mice. The exon 2~41 and flanking sequences (~0.7 kb upstream of exon 2 to ~0.9 kb downstream of 3’UTR) of mouse Hc were replaced with the exon 2~41 and flanking sequences of human C5 (~0.7 kb upstream of exon 2 to ~1.5 kb downstream of 3’UTR). The murine exon 1 coding region [aa.1~21, containing signal peptide (aa.1~18)] was kept.
응용 분야
검증 데이터
1. Normal retinal morphology in B6-hC5 mice
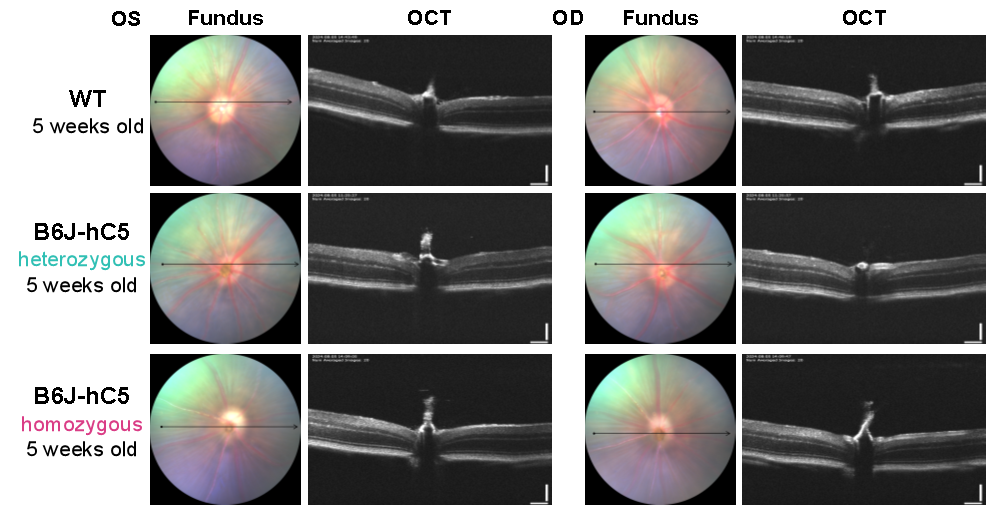
Figure 2. Fundus morphology and OCT results of WT and B6J-hC5 mice. The fundus morphology and retinal OCT results of heterozygous and homozygous B6J-hC5 mice were consistent with those of wild-type.
2. Normal retinal photoreceptor function in B6-hC5 mice
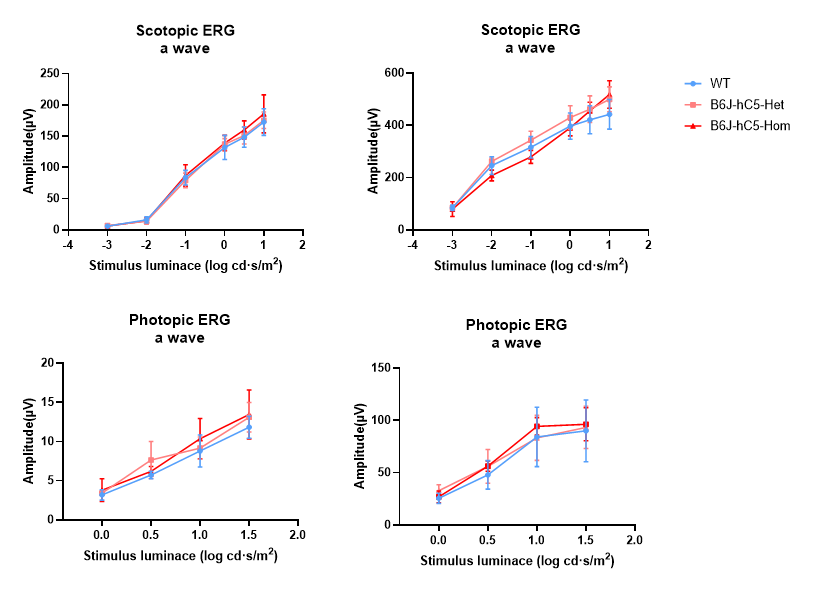
Figure 3. Electroretinogram (ERG) detection results of WT and B6J-hC5 mice. Compared with WT, the amplitudes of the a-wave and b-wave in both scotopic and photopic ERG recordings of heterozygous and homozygous B6J-hC5 mice were nearly identical to those of the WT. The retinal photoreceptor function of heterozygous and homozygous B6J-hC5 mice was normal.
참고 문헌