

Researchers studied the mechanisms of MARCHF2 by generating corresponding allelic variations of Marchf2 mice. As we know, many monumental findings have small beginnings. To this end, we introduce gene research' s great example of this – Marchf2 knockout mice.
Marchf2 Gene Knockout Mice
NF-kappaB essential modulator (NEMO) is a key regulatory protein that functions during NF-kappaB- and interferon-mediated signaling in response to extracellular stimuli and pathogen infections. Tight regulation of NEMO is essential for host innate immune responses and for maintenance of homeostasis. Researchers report that the E3 ligase MARCHF2 is a novel negative regulator of NEMO-mediated signaling upon bacterial or viral infection, described as follows. MARCHF2 interacted directly with NEMO during the late phase of infection and catalyzed K-48-linked ubiquitination of Lys326 on NEMO, which resulted in its degradation. Deletion of MARCHF2 resulted in marked resistance to bacterial/viral infection, along with increased innate immune responses both in vitro and in vivo. In addition, MARCHF2(-/-) mice were more susceptible to LPS challenge due to massive production of cytokines. Taken together, these findings provide new insight into the molecular regulation of NEMO and suggest an important role for MARCHF2 in homeostatic control of innate immune responses.[6]
Generation of Marchf2 Knockout Mice
In order to explore the specific immunomodulatory function of MARCHF2 protein upon viral or bacterial infections, researchers used March2 knockout mice to demonstrate the physiological role of MARCH2. Resultsa showed that MARCH2 is a negative regulator of the NF-κB and type I IFN signaling pathways upon viral or bacterial infection.
In this study, female mice (C57BL/6J, 4–6 weeks old) were used as embryo donors for superovulation. After mating, resultant fertilized embryos were collected from the oviducts and cultured in KSOM medium. Microinjection was performed using an Olympus IX71 Inverted Microscope with the Narishigi microinjection system. gRNA (50 ng/μl) and Cas9 mRNA (100 ng/μl) were mixed and injected into the cytoplasm of the zygotes with visible pronuclei in M2 medium. The injected embryos were cultured overnight in KSOM medium at 37°C with 5% CO2. Embryos at the two-cell stage were transferred into oviducts of pseudopregnant ICR females. Tail samples from 3-week-old live pups were collected for extraction of genomic DNA and sequenced. Presumptive heterozygous March2+/− were mated. To screen March2 KO mice, T7E1 assays were performed using genomic DNA samples from tail biopsies. Briefly, the genomic region included RNA-guided endonucleases target site was PCR-amplified with nested PCR method, melted, and reannealed to form heteroduplex DNA, which was treated with T7 endonuclease 1 (New England Biolabs) and then analyzed by agarose gel electrophoresis. Once heterogeneous mice were excluded, additional T7E1 assay was conducted by mixing equal amounts of wild-type PCR products and WT and KO mice were separated by agarose gel electrophoresis (Fig 1.G), confirming the March2 gene knockout.
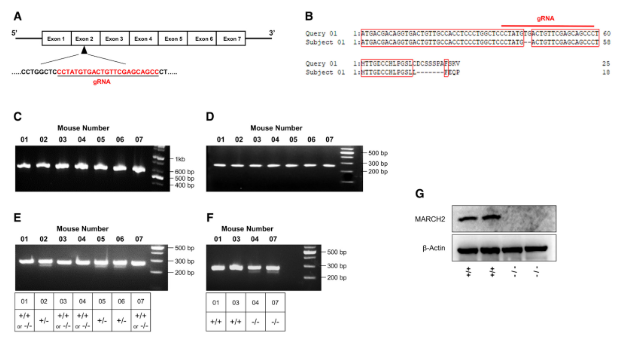
Figure 1. MARCH2 knockout mice generation [6]
MARCHF2 plays a critical role in host defense in vivo
To evaluate the potential roles of MARCHF2 in innate immune responses, researchers generated MARCHF2−/− mice on a C57BL/6 background using the CRISPR-Pro system and verified genetic knockout of the MARCH2 gene (Fig 1A–G). MARCHF2−/− mice did not show any external abnormalities.
First, to investigate the important role of MARCHF2 in viral infection in vivo, they injected MARCHF2+/+ and MARCHF2−/− mice with VSV-GFP intravenously (Fig 2A). The serum of MARCHF2−/− mice contained fewer replicating viruses than that of MARCHF2+/+ mice. In addition, when researchers measured the amount of cytokines in the serum, they found that the concentrations of IFN-β, IL-6, IL-12, and TNF-α were higher in MARCHF2−/− mice than in MARCHF2+/+ mice (Fig 2B). Furthermore, they measured serum cytokine levels in mice injected with the viral RNA mimic ligand, poly(I:C) (Fig 2C). As observed for virus infection, serum cytokine levels in MARCHF2−/− mice after ligand stimulation were higher than those in MARCHF2+/+ mice. These results strongly suggest that MARCHF2 is involved in antiviral innate immune responses in vivo.
Second, to investigate whether MARCHF2 plays a role in response to bacterial infection, researchers challenged MARCHF2+/+ and MARCHF2−/− mice with a lethal dose of Listeria monocytogenes (Lm). As shown in Fig 2D and E, MARCHF2−/− mice were more resistant to bacterial infection than MARCHF2+/+ mice. To examine the effects on pathogen clearance, they examined the bacterial burden in the spleen and liver using colony-forming unit (CFU) titration assays (Fig 2F and G). The results correlated with those of the survival assay; the bacterial load in MARCH2−/− mice was significantly lower than that in MARCHF2+/+ mice. Next, they investigated whether the low bacterial burden in MARCHF2−/− mice was due to higher inflammatory responses in these animals. Serum, spleen, and liver samples from MARCH2−/− mice contained higher levels of IL-6, IL-12, CCL5, and CXCL10 than those from MARCHF2+/+ mice (Fig 2H–J). These results also indicate that MARCHF2 is involved in antibacterial innate immune responses in vivo, with a deficiency of MARCH2 strengthening antimicrobial immunity.
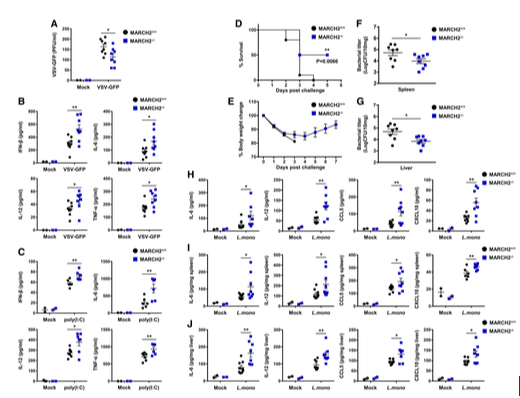
Figure 2. Deficiency of MARCH2 strengthens antimicrobial immunity in vivo A, B.MARCH2+/+ (n = 8) or MARCH2−/− (n = 8) mice were intravenously infected with VSV-GFP (2 × 108 PFU/mouse), and serum samples were collected at 12 h post-infection (hpi). (A) Virus titer was analyzed in a plaque assay. (B) Secretion of IFN-β, IL-6, IL-12, and TNF-α was measured in specific ELISAs. C.MARCH2+/+ (n = 6) or MARCH2−/− (n = 6) mice were intravenously injected with poly(I:C) (200 μg/mouse). Serum samples were collected at 2 hpi, and secretion of IFN-β, IL-6, IL-12, and TNF-α was measured in specific ELISAs. D–J.MARCH2+/+ (n = 8) or MARCH2−/− (n = 8) mice were intraperitoneally challenged with 1 × 106 CFU of Listeria monocytogenes. (D) The percentage of surviving mice (D, log-rank test, **P < 0.01) and body weight changes for each group (E) are shown. (F, G) At 3 dpc, the bacterial load in the spleen (F) and liver (G) was examined. (H–J) Amount of IL-6, IL-12, CCL5, and CXCL10 in serum (H), spleen (I), and liver (J) of mice in each group at 24 hpc. Data information: *P < 0.05, **P < 0.01 (two-tailed Student's t-test). Data are expressed as the mean ± SEM. [6]
Third, to investigate whether MARCHF2 is responsible for susceptibility to lipopolysaccharide (LPS)-induced septic shock in vivo, MARCH2+/+ and MARCHF2−/− mice were injected intraperitoneally with LPS. All MARCH2+/+ mice survived, whereas over 60% of MARCHF2−/− mice died (Fig 3A and B). These results suggest that MARCHF2−/− mice are more susceptible to endotoxin shock than MARCHF2+/+ mice. Furthermore, hematoxylin and eosin staining of spleen sections revealed greater inflammatory cell infiltration in MARCHF2−/− mice than in MARCHF2+/+ mice (Fig 3C). Therefore, they hypothesized that MARCHF2 is involved in inflammatory responses during LPS-mediated septic shock. To test this, they used an ELISA to measure cytokine levels in serum (Fig 3D). As expected, MARCHF2−/− mice showed markedly higher expression of inflammatory mediators than MARCHF2+/+ mice. Researchers also measured expression of mRNA encoding pro-inflammatory cytokine/chemokine genes in the spleen and liver. As shown in Fig 3E and F, expression of mRNA encoding IL-6, TNF-α, CXCL10, and IL-1β was significantly higher in MARCHF2−/− mice than in MARCHF2+/+ mice. These data indicate that MARCHF2 attenuates inflammatory responses under conditions of excessive inflammation. Collectively, the results provide in vivo evidence that MARCHF2 is a critical regulator in innate immune responses against viral and bacterial infection.
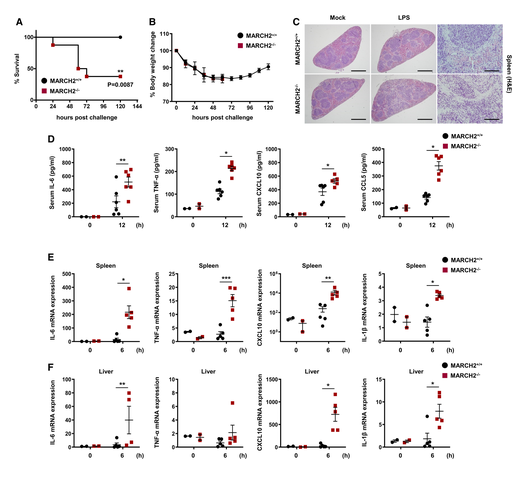
Figure 3. Deficiency of MARCH2 induces susceptibility to LPS-induced septic shock A, B.MARCH2+/+ (n = 8) or MARCH2−/− (n = 8) mice were challenged intraperitoneally with LPS (24 mg/kg). (A, B) Percentage of surviving mice (A, log-rank test, **P < 0.01) and body weight changes (B) in each group. C.MARCH2+/+ (n = 4) or MARCH2−/− (n = 4) mice were challenged intraperitoneally with LPS (24 mg/kg). Representing slides of H&E staining of spleen sections from each group. Scale bar, 6 mm. D.MARCH2+/+ (n = 6) or MARCH2−/− (n = 6) mice were challenged intraperitoneally with LPS (24 mg/kg). Levels of IL-6, TNF-α, CXCL-10, and CCL-5 in serum from mice in each group were measured at 12 hpc by ELISA. E, F.MARCH2+/+ (n = 5) or MARCH2−/− (n = 5) mice were challenged intraperitoneally with LPS (24 mg/kg). cDNA was prepared from total RNA extracted from spleen and liver of mice. Expression of mRNA encoding IL-6, TNF-α, CXCL10, and IL-1β in spleen (E) and liver (F) from mice in each group was examined at 6 hpc by qPCR. Data information: *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student's t-test). Data are expressed as the mean ± SEM. [6]
Summary: Future Research
As described, Marchf2 interacts with NF-κB regulatory factor (NEMO) in the later stages of infection and further activates the expression of inflammatory pathway related factors. Genetic knockout of Marchf2 increased the resistance of mice to bacterial and viral infections, enhancing the innate immune response. Since Marchf2 plays an important role in virus infection, is it also a potential target in the development of new drugs for COVID-19 or future viral outbreaks?
References:
1.https://www.ncbi.nlm.nih.gov/gene/51257
2.https://www.uniprot.org/uniprot/Q9P0N8#function
3.https://alphafold.ebi.ac.uk/entry/Q9P0N8
4.https://www.genecards.org/cgi-bin/carddisp.pl?gene=MARCHF2&keywords=Marchf2
5.http://www.informatics.jax.org/marker/MGI:1925915
6.Chathuranga K, Kim TH, Lee H, Park JS, Kim JH, Chathuranga WAG, Ekanayaka P, Choi YJ, Lee CH, Kim CJ, Jung JU, Lee JS. Negative regulation of NEMO signaling by the ubiquitin E3 ligase Marchf2. EMBO J. 2020 Nov 2;39(21):e105139. doi: 10.15252/embj.2020105139. Epub 2020 Sep 16. PMID: 32935379; PMCID: PMC7604578.




영업일 기준 1-2일 내에 답변해 드리겠습니다.