

On October 6 of last year, Voyager Therapeutics announced a cooperation agreement with Pfizer. Pfizer will have the option to license novel capsids generated by Voyager's TRACER capsid platform to develop gene therapies for neurological and cardiovascular diseases. Voyager will receive 30 million dollars in advance and up to 580 million dollars in development, supervision, and commercial milestones. With the rapid increase in gene therapy research, the application of adeno-associated viral (AAV) vector technology in the field of gene therapy has become mature, and a number of new companies such as Voyager Therapeutics have emerged as major multinational pharmaceutical giants have also begun to deploy in this field.
As of this year, there have been hundreds of recombinant adeno-associated virus (rAAV) gene therapy products approved to be marketed and entered into clinical trials worldwide. According to the official website of Clinical Trials, there are a total of 270 AAV-related clinical trials worldwide. On June 28, 2021, NR082-China's first clinical-stage ophthalmic rAAV in vivo gene therapy drug (for Leber Hereditary Optic Neuropathy (LHON) caused by ND4 mitochondrial gene mutation) completed the first patient enrollment and administration in China. Collectively, the data shows that the research and development of AAV in vivo gene therapy products has entered the fast lane.
Why does AAV vector have such potential? This is related to the characteristics of the AAV vector itself, which are described below.
The Genome and Characteristics of AAV
The genome of the adeno-associated virus (AAV) is a single-stranded DNA fragment of about 4.7 kb, contained in an icosahedral non-enveloped virus capsid with a diameter of 20 nm. The genome of AAV consists of three functional modules: two open reading frames (ORFs) - which include the replication (Rep) and capsid (Cap) genes - and the inverted terminal repeat (ITR).Rep genes encode four proteins required for virus replication; they are named after their molecular weights: Rep78, Rep68, Rep52 and Rep40.
The Cap genes encode three capsid proteins through alternative splicing and translation of different start codons, namely VP1, VP2 and VP3. The difference in the capsid protein sequence makes AAV produce different serotypes. Different serotypes of AAV can bind to the surface receptors of different cells, resulting in AAV carriers which can target different tissues.
Figure 1. Schematic diagram of the AAV virus structure [8]
By engineering AAV, scientists have produced a recombinant AAV vector (rAAV) suitable for cell transfection. rAAV is composed of the same capsid sequence and structure as wild-type AAV, but the difference is that all AAV protein coding sequences are deleted from the genome of rAAV packaging, and a therapeutic gene expression cassette is added. The only virus-derived sequence is ITR, which is necessary for directing genome replication and packaging during vector production. The complete removal of the viral coding sequence maximizes the packaging capacity of rAAV and contributes to their low immunogenicity and cytotoxicity when delivered in vivo.
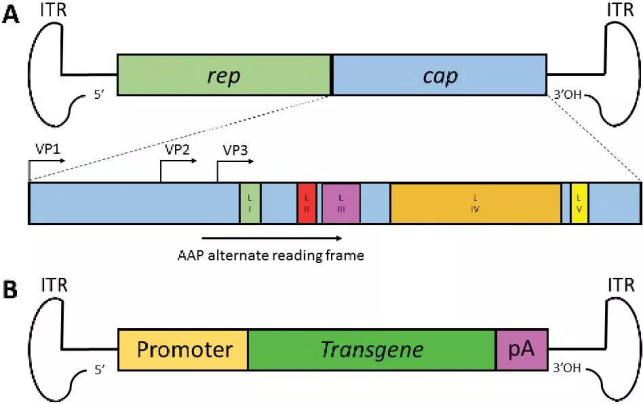
Figure 2. The genome structure of wild AAV and rAAV [10]
Compared with other virus-based research tools, AAV has many advantages, making it the most widely used in vivo gene therapy viral vector. The advantages of AAV vectors for gene therapy applications include:
1. Safety. Wild-type AAV has never been found to be pathogenic to humans (80% of people have been infected with AAV), and recombinant AAV has removed 96% of the wild-type AAV genome, further ensuring safety.
2. Stable expression. AAV can express foreign genes stably for a long time in non-dividing cells.
3. Highly targeted. AAV has strong targeting ability and can be specifically transfected to target organs and tissues.
4. Wide host range. Able to transduce among dividing cells and also cells in the quiescent phase.
5. Stable physical properties. rAAV cannot be inactivated even at 60 degrees Celsius, and it is resistant to chloroform.
Application of AAV in Cardiovascular Disease
The current clinical applications of rAAV in vivo gene therapy are mainly concentrated in the fields of rare diseases, ophthalmic diseases, metabolic diseases, cardiovascular diseases, and neurological diseases. Today, we will mainly introduce its applications in cardiovascular disease research.
The global clinical trials of rAAV in cardiovascular diseases are mainly focused on the following indications. The product layout of some related companies is shown in Table 1.
|
Disease name |
Phase |
Drug Name |
Company |
|
Heart failure |
I I/II |
NAN-101 MYDICAR |
AskBio Celladon Corporation |
|
Cardiomyopathy |
I/II |
MYDICAR |
Celladon Corporation |
|
Homozygous familial hypercholesterolemia |
I/II |
N/A |
Regenxbio |
|
Danon disease |
I |
RP-A501 |
Rocket Pharmaceuticals |
Table 1. Clinical progress of AAV gene therapy for cardiovascular diseases.
1. Heart failure
These six clinical trials all deliver the SERCA2a gene to heart tissue through rAAV. Abnormal calcium circulation is a common feature of all types of heart failure. SERCA2a plays a central role in maintaining the calcium circulation of the heart. In patients with heart failure, the expression of SERCA2a decreases. By restoring the expression of SERCA2a, heart function can be improved.
2. Cardiomyopathy
Ischemic cardiomyopathy (ICM) refers to the localized or diffuse fibrosis of the myocardium due to long-term myocardial ischemia, resulting in impaired systolic and/or diastolic function of the heart, causing the heart to expand and become stiff, and may result in congestive heart failure (CHF). Heart strength decreases along with this series of clinical syndromes, with the earliest symptoms including arrhythmia. There are currently three clinical trials in the world for rAAV treatment of cardiomyopathy, which are mainly achieved through the delivery of SERCA2a.
3. Homozygous familial hypercholesterolemia
Familial hypercholesterolemia (FH) is an inherited disease caused by mutations in one of the key genes of low density lipoprotein cholesterol (LDL-C) catabolism. At present, there are two clinical studies in the world for familial hypercholesterolemia, which play a role by delivering the correct low density lipoprotein receptor (LDLR) gene. LDLR plays an important role in the normal metabolism and transportation of cholesterol.
4. Danon disease
Danon Disease is an X-linked dominant genetic disease, which results in more severe pathological effects among males.Due to mutations in the lysosomal-associated membrane protein 2 (LAMP2) gene, the patient's skeletal muscle and cardiac muscle were weakened, resulting in multiple organ disorders, and eventually resulting in severe heart failure if they do not receive a heart transplant.
In March last year, researchers from the University of California San Diego School of Medicine published an article in "Science Translational Medicine". They first constructed a mouse model that knocked out the LAMP2 gene, and then delivered the LAMP2B gene to the whole body of the mouse through systemic injection of rAAV9. Data showed that LAMP2B can restore protein expression in multiple organs of mice, improve metabolic abnormalities and cardiac function, and increase survival rate. These results indicate that gene therapy delivery of LAMP2B may be a treatment option for patients with Danon's disease, and related clinical trials are already underway.
The Challenges of AAV in Disease Applications
Although AAV has broad potential in the treatment of metabolic and cardiovascular diseases, it also has certain limitations that require our attention:
1. AAV can only hold small gene fragments: it can only accommodate genes under 5.0kb, and many genes are much larger than 5.0kb.
2. The expression is not stable enough: because rAAV will not be integrated into the host genome, it will gradually be lost after transfection due to mitosis.
3. Immune system interactions: First, many people already have AAV neutralizing antibodies in their bodies. The second is that rAAV protein capsids and genomic protein products can interact with the host immune system at multiple stages, posing obstacles to effective gene delivery and durable gene expression.
Summary
The rAAV vector is currently the first choice for in vivo gene therapy due to its many advantages. At present, three rAAV products have been approved for marketing in Europe and the United States, which are used to treat neurological diseases and ophthalmological diseases.There have yet to be any rAAV products related to treating cardiovascular diseases approved in Europe and the United States. However, there are a large number of clinical trials exploring rAAV research and therapeutic applications in cardiovascular disease. It is believed that in the near future,more products will be approved for marketing, providing hope for the treatment of intractable metabolic-related diseases.
References:
1. https://clinicaltrials.gov/
2. https://www.fda.gov/
3. https://www.ema.europa.eu/
4. Nguyen GN,Everett JK,Kafle S,Roche AM,Raymond HE,Leiby J,Wood C, Assenmacher CA,Merricks EP,Long CT,Kazazian HH,Nichols TC,Bushman FD, Sabatino DE. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat Biotechnol. 2021 Jan;39(1):47-55. doi: 10.1038/s41587-020-0741-7.Epub 2020 Nov 16.PMID: 33199875; PMCID: PMC7855056.
5. Shi H, Xue T, Yang Y, Jiang C, Huang S, Yang Q, Lei D, You Z, Jin T, Wu F, Zhao Q, Ye X. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease.Sci Adv.2020 Jun 17;6(25):eaaz3621.doi:10.1126/sciadv.aaz3621.PMID: 32596444; PMCID: PMC7299628.
6. Hammoudi N, Ishikawa K, Hajjar RJ. Adeno-associated virus-mediated gene therapy in cardiovascular disease.Curr Opin Cardiol.2015 May;30(3):228-34.doi: 10.1097/HCO.0000000000000159. PMID: 25783685; PMCID: PMC4417622.
7. Cao G, Xuan X, Zhang R, Hu J, Dong H. Gene Therapy for Cardiovascular Disease: Basic Research and Clinical Prospects. Front Cardiovasc Med. 2021 Nov 5;8:760140. doi: 10.3389/fcvm.2021.760140. PMID: 34805315; PMCID: PMC8602679.
8. Lugin ML, Lee RT, Kwon YJ. Synthetically Engineered Adeno-Associated Virus for Efficient, Safe,and Versatile Gene Therapy Applications. ACS Nano. 2020 Nov 24;14(11):14262-14283. doi: 10.1021/acsnano.0c03850. Epub 2020 Oct 19.PMID: 33073995.
9. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019 May;18(5):358-378.doi: 10.1038/s41573-019-0012-9. PMID: 30710128; PMCID: PMC6927556.
10.Balakrishnan B, Jayandharan GR.Basic biology of adeno-associated virus (AAV)vectors used in gene therapy.Curr Gene Ther.2014;14(2):86-100. doi: 10.2174/1566523214666140302193709. PMID: 24588706.
11. Bera A, Sen D. Promise of adeno-associated virus as a gene therapy vector for cardiovascular diseases.Heart Fail Rev.2017 Nov;22(6):795-823.doi: 10.1007/s10741-017-9622-7. PMID: 28589503.




영업일 기준 1-2일 내에 답변해 드리겠습니다.