

The suicidal response of Immune system to the SARS-CoV-2
Since COVID-19 poses a significant threat to public health and also represents a challenge for antiviral drug development and administration globally, current efforts focus on prevention and control while accelerating the research on the SARS-CoV-2 coronavirus, which has caused the outbreak of COVID-19. So far, studies on the epidemiological and clinical characteristics of this illness is still growing, but there is significant anecdotal evidence of value. Most patients suffering from COVID-19 were observed with symptoms like fever, cough, and diarrhea, but also some express serious complications such as acute respiratory failure, acute respiratory distress, and septic shock. The "COVID-19 Diagnosis and Treatment Program (6th Edition)" issued by the National Health Commission of the People’s Republic of China, mentioned the clinical warning indicators for the diagnosis and treatment of patients with severe and critical illness: "Severe and critically ill patients often presents with elevated inflammatory factors." One of the main causes of SARS-CoV-2-related death is the cytokine storm caused by the excessive activation of the human immune system.
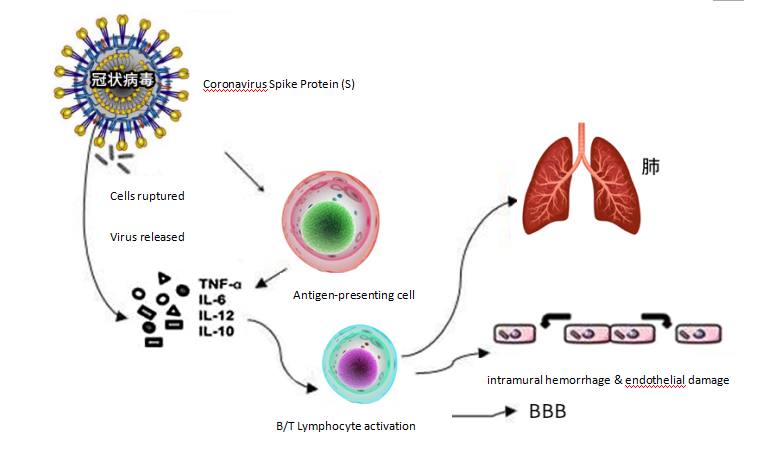
Graph 1. The immune process of coronavirus invasion and its organism damage
Cytokine Storm (a.k.a. hypercytokinemia) refers to the rapid and large-scale production of multiple cytokines in body fluids (such as TNF-α, IL-1, IL-6, IL-12, IFN-α, IFN-β, IFN-γ, MCP-1 and IL-8) after the body is infected with microorganisms, which is academically called ‘Cytokine Storm Syndrome (CSS)’. CSS is a severe life-threatening syndrome, marking an uncontrolled and dysfunctional immune response that involves the continued activation and expansion of lymphocytes and macrophages, which secrete large amounts of cytokines, and may cause Systemic Inflammatory Response Syndrome (SIRS), Multisystem Organ Failure (MSOF), Methemoglobinemia (MetHb), Acute respiratory distress syndrome (ARDS), and other diseases.
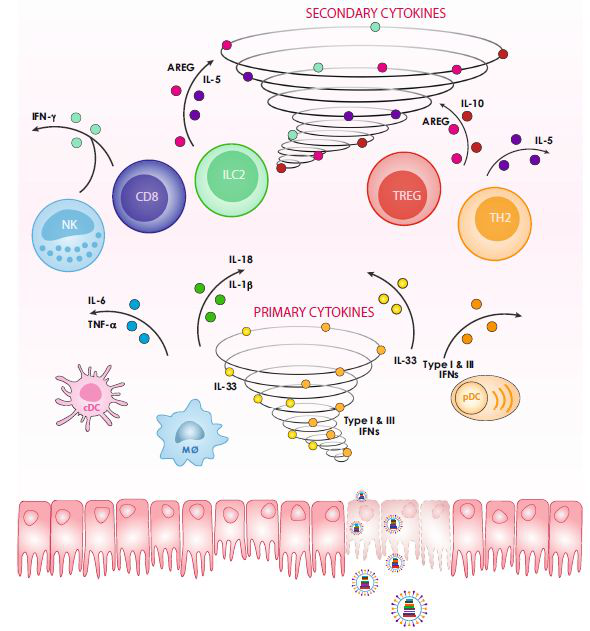
Graph 2. The Genesis Process of Cytokine Storm after Infection
As we all know, the immune system is vital for the body to perform both immune responses and functions, which has the role of identifying and eliminating antigenic foreign bodies, coordinating with other systems of the body, and jointly maintaining the stability of the body's environment and physiological balance. Under normal circumstances, the immune system helps the body to clear the infection, but once the immune system is activated to the point of being out-of-control, such as in CSS, it will harm the host and attack the body violently. Cytokines are one of the important barriers used by the human immune system to fight against exogenous infections when pathogens invade human cells. The cytokine storm is a signal for help, with the aim to trigger the immune system function and induce apoptosis of the cells - thereby causing great harm to host in the process of destroying the pathogen. The first organ system being harmed is the cardiovascular system, more specifically, blood vessels. Cytokine storms thin the vascular walls - making them easier to penetrate - and arteries, veins, and capillaries begin to exude plasma. At the same time, a large amount of Nitric Oxide (NO) is released due to cytokine storms, which further damages blood vessels and causes septic shock. In addition, in an excessive immune response, immune cells attack the body's own cells to cause multiple organ failures, such as in the heart, kidney, and lung.
Table 1. The Cytokine Changes Caused by CSS and their Related Complications
| Complications caused by CSS | Changes in Cytokines compared to normal (healthy) human |
| Hepatic Failure | TNF-α, IL-6, IL-10, IL-17, etc. increased significantly, among which IL-6 and TNF-α increased with the severity of the disease |
| Kidney Disease | IL-1, IL-6 and TNF-α increased significantly |
| Heart Failure | IL-6 and TNFα increased; IL-10 decreased |
| Coagulation System Disorders | There is a close but complex link between cytokines and coagulation factors |
Huang KJ et al. (2005) proposed that SARS-CoV infection can induce a large number of IFN-γ-related cytokine storms, which may be involved in the immunopathological damage seen in SARS patients. A study in 2006 by Jan-Inge Henter et al. has found that patients with H5N1 infection will clinically develop both hematocytosis and leukocytosis alongside Acute Encephalitis Syndrome (AES). In 2013, a research team mentioned that serum samples from patients with H7N9 acute infections presented elevated levels of cytokines IP-10, MIG, MIP-1β, MCP-1, IL-6, IL-8 and IFN-α. This evidence collectively indicates that CSS is one of the causes of high mortality in critical, high-risk patients after viral infection.
Cytokines are low molecular weight soluble proteins produced by various cells upon induction by immunogens, mitogens, or other stimulants. They have the ability to regulate innate and adaptive immunity, hemocytogenesis, cell growth, APSC (adult pancreatic stem cells), pluripotent cells, repair of damaged tissues, and additional processes. Cytokines can be divided into several categories, including: interleukins (ILs), interferons (IFNs), Tumor Necrosis Factor (TNF) superfamily, Colony-stimulating factors (CSFs), Chemokines, growth factors, and more. In this section, we will take interferon as an example to explore how cytokines play a role in the immune system of a virus-infected body.
Interferons (IFNs) are a primary line of defense against viral infection, inducing an antiviral response within the host that helps to impede viral pathogenesis. One example is plasmacytoid dendritic cell-derived type I IFN, which is essential for regulating virus-induced cytopathy. However, the broad range and diversity of interferon-stimulating genes (ISGs) poses eminent challenges to virus pathogenesis research. Vineet D. Menachery et al. in 2014 analyzed the ISG response in pathogenic influenza and SARS coronavirus - transcriptomics and proteomics datasets were used to compare the ISG response patterns following highly pathogenic H5N1 avian influenza (HPAI) A virus, 2009 pandemic H1N1, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and Middle East respiratory syndrome CoV (MERS-CoV) infection, which revealed that the interferon-induced response varied among the viruses tested. Through the combination of systems biology and experimental verification, researchers have identified the strategy used by the virus to circumvent the immune response; they discovered that not only were antagonistic effects caused by the IFN-I signaling delays, but also that the virus exerted control over the pathway of immune response via altered histone modification. This delayed IFN-I signaling promotes the accumulation of pathogenic inflammatory monocyte-macrophages (IMMs), leading to increased level of lung cytokine/chemokines, vascular leakage, and impaired virus-specific T cell responses. Notably, the depletion of IFN-α / β receptors (IFNAR) or IMM protects mice from lethal infections, without affecting viral load. These results demonstrate that IFN-I and IMM contribute to lethal SARS-CoV infection in patients and perhaps other respiratory viruses; therefore, IFN-I and IMM can be potential therapeutic targets. Yohichi Kumaki et al. in 2011 demonstrated the protective and therapeutic function of the IFNα gene in a mouse model of SARS-CoV infection. Additional research by Lisa E. Hensley et al. found that recombinant human interferon (IFN)-β 1a potently inhibits SARS coronavirus replication in vitro.
The above-noted research illustrates the various ways in which the virus attacks the host from the perspective of cytokines. While we know that lung disease is the most important feature of infection, there are additional effects on the body as a unified system. Just as in ‘The Butterfly Effect’, not only the infection in lungs, but the entire range of sequelae stemming from viral infections should be taken into consideration. The sudden invasion of SARS-CoV-2 has caught the world off guard. With the rapid advancement of science and technology and the improvement of prevention and control measures, we are increasing both our scientific understanding and public awareness about viruses. In the course of combating this pandemic, the emerging achievements and results could be the powerful weapons in fighting COVID-19 and preventing future outbreaks.
Since the outbreak, the R&D team at Cyagen has made every effort to develop animal models catered to the global SARS-CoV-2 research initiative. Additionally, we have launched the public welfare project exclusively for the front-line scientific researchers participating in the epidemic rescue in Hubei Province, China - where COVID-19 was first discovered and rapidly became the most severely afflicted area. Cyagen can provide customized knockout (KO), knockin (KI), conditional knockout (cKO), point mutation, or humanized mouse models cater to your research needs - from coronavirus receptor targets to factors in other cellular processes, our experts will provide you with an optimized gene modification strategy. For consultation and order, please contact us.
Online reservations on COVID-19 ACE2 & DPP4 animal models open NOW!
● C57BL/6 strain인 ACE2 및 DPP4 Humanized 마우스, Knock-Out 마우스, Rosa26 Knock-In 마우스 모델을 주문 시, 다음과 같은 할인 혜택을 받을 수 있습니다.
| 서비스 | 상세 내역 | 할인 가격(USD/Strain) | |
|---|---|---|---|
| ACE2 / DPP4 | Knock-Out 마우스 | F1 Heterozygous 마우스 ≥ 3마리 | $3,599 |
| Humanized 마우스 | F1 Heterozygous 마우스 4마리 | $5,000 | |
| Knock-In 마우스 (Rosa26) | F1 Heterozygous 마우스 4마리 | $6,000 | |
● ACE2,DPP4 외에 코로나바이러스감염증 연구와 관련된 다른 마우스 모델에 대한 수요가 있으신다면 코로나바이러스감염증 연구 관련 증명 자료를 Cyagen에 제공해 주시면 Cyagen 전문가팀에서 평가 진행 후, 할인 혜택을 받을 수 있습니다.
● Cyagen에서 BALB/c strain의 유전자 편집 마우스도 제공해 드릴 수 있습니다. 관심이 있으시면 연락 부탁드립니다.
References:




영업일 기준 1-2일 내에 답변해 드리겠습니다.