

Mouse Embryonic Fibroblasts (MEF) are widely used as feeder cells for supporting the growth of both mouse and human embryonic stem cells (ESCs). By providing a suitable attachment substrate and critical soluble factors for ESCs, MEF help to maintain ESC stemness {McElroy, 2008 #1}.
Characteristics:
- Neomycin (≥200μg/ml), Hygromycin (≥60μg/ml), and Puromycin (≥0.4μg/ml) resistant;
- γ-irradiated to arrest mitotic growth;
- Validated to support growth of 6 different ES cell lines that are used in Cyagen’s in-house knockout and knockin mouse projects;
- Greater than 80% post-cryostorage viability.
Validation Experiments:
Each batch of Cyagen KO-certified 3R MEF (irradiated) is tested to support growth of 6 different ES cell lines that have been successfully used in Cyagen’s in-house gene targeting projects to generate chimeras and germline F1 heterozygous mice.
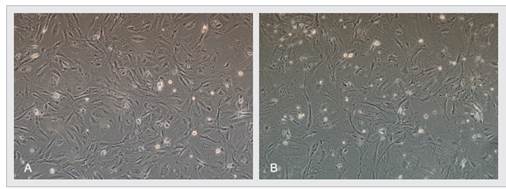
Figure 1. Cyagen OriCell™ KO-certified 3R MEFs (irradiated, P1) (A) and MEFs from competing brand of the same passage number (B) are cultured in vitro post-thaw and visualized via optical microscopy (100×). As indicated by the number of apoptotic bodies, Cyagen KO-certified 3R MEFs have greater post-cryopreservation viability than the competing product and display healthier cell morphologies.
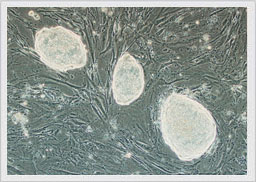
Figure 2. Gene targeting vectors are electroporated into mouse embryonic stem cells (mESC) grown on Cyagen OriCell™ KO-certified 3R MEFs, and mESCs are visualized via optical microscopy 6 days after selection on MEF feeder cells (100×). As shown in the image, mESCs expand rapidly to form tightly-packed, spherical colonies with smooth membrane surfaces and rounded nucleus.
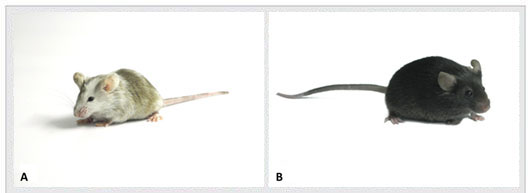
Figure 3. Mouse embryonic stem cells cultured on Cyagen OriCell™ KO-certified 3R MEFs are electroporated with gene targeting vectors and subsequently injected into host blastocysts followed by transfer into surrogate mothers to produce male chimera mice (A). Germline transmission F1 mutants (B) are obtained by breeding male chimera mice to female mice of matching genetic background.
Quality Control:
Tested negative for bacteria, fungi and mycoplasma.
Delivery and Storage:
- Cells are shipped in the format of cryovials on dry ice and should be stored in liquid nitrogen immediately upon receipt. However, upon receipt, if no dry ice is left in the package, please freeze the cells down at -80°C until use to maximize cell viability.
- Cells must be maintained at -80°C until use and prolonged exposure to room temperature may be detrimental to cell viability. Limit thawing process to no more than 3 minutes to prevent potential toxic effects of DMSO on post-cryostorage cell survival rate.
- When stored at the recommended conditions, components are stable up to the expiration date listed on each product label.
Product Warranty:
Cyagen warrants its cells only if suggested media are used and the recommended protocols are followed. Cryopreserved fibroblasts are guaranteed viable and functional when thawed and maintained as directed in product user manual.
Intended Use:
Intended for non-human laboratory research use only.
Reference:
McElroy, S. L. and R. A. ReijoPera (2008). Preparation of Mouse Embryonic Fibroblast Feeder Cells for Human Embryonic Stem Cell Culture. 2008: pdb.prot5041.