

Humans are estimated to have upwards of 20,000 protein-coding genes, each of which play a unique functional role in our biology. Considering the various mutations that may be present across genetic loci as well as the interplay of these factors, it may quickly become an overwhelming topic to review. To support researchers looking for regular information on genetic targets of interest, we have developed our Weekly Gene series to introduce the development trend and future direction of research across human genes. Cyagen’s Weekly Gene series provides insights into popularly researched genes, their basic information, research overview, and application background of the gene in detail. This week’s subject is the ERCC6 gene, which is closely related to the development of Cockayne syndrome B (CSB).
Introduction to ERCC6 gene
Mutations in the excision and repair of cross-complementation group 6 (ERCC6) gene is known to result in Cockayne syndrome B (CSB), among some other disorders. This gene encodes a DNA splicing repair protein, which has ATP-stimulated ATPase activity, interacts with several transcription and excision repair proteins, and may promote the formation of complexes at DNA repair sites, in transcription coupled with nucleotide excision repair (TC-NER) plays an important role. The ERCC6 gene is located on human chromosome 10, 10q11, with a total length of 104.7 kb, a total of 23 exons, and 1493 amino acids. Mutations in the ERCC6 gene are closely related to Cockayne syndrome B (CSB).
|
Species |
Human |
Mouse |
Rat |
|
Chromosome |
10 |
14 |
16 |
|
Full Length (bp) |
104658 |
67700 |
70605 |
|
mRNA (nt) |
8,993 |
8422 |
7003 |
|
Numbers of exons |
23 |
23 |
23 |
|
Numbers of amino acids |
1493 |
1481 |
1325 |
|
Gene Family |
SMARCA1, SMARCA5, CHD5, CHD7, CHD2 |
||
|
Related Disease |
Cockayne Syndrome |
||
ERCC6 and Cockayne Syndrome (CS)
Cockayne syndrome (CS) is a rare autosomal recessive genetic disease, with an incidence of 1/2.77 million and 1/2.70 million in Japan and Western Europe, respectively. The clinical features of CS are complex and diverse, including growth retardation, premature aging, retinopathy pigmentosa, light sensitivity, sensorineural hearing loss, and microcephaly. Its clinical phenotype is a continuous and overlapping spectrum, from the most serious to the brain-eye-facial-bone syndrome (COFS), CS Ⅱ, CS Ⅰ, CS Ⅲ, and ultraviolet sensitive syndrome.
Currently, there are three main methods for CS diagnosis:
(1) Diagnosis is based on typical phenotypes, and the specific diagnostic criteria are the version revised by Laugel in 2013 shown in Figure 1. However, this method is only suitable for CS in a narrow sense, namely CS Ⅰ-Ⅲ, and not for COFS and ultraviolet sensitive syndrome.
(2) Based on the theory that CS has defects in TC-NER, conduct a specific DNA repair test to measure the repair of RNA synthesis after skin fibroblasts are exposed to ultraviolet radiation.
(3) Use next-generation sequencing technology for DNA or RNA sequencing analysis for genetic diagnosis [1].
Mutations in ERCC6 (CSB) and ERCC8 (CSA), causing CSB and CSA respectively, are the main pathogenic genes of CS, of which CSB gene mutation accounts for about 2/3. CSA and CSB participate in the repair of damaged DNA through the TC-NER pathway. CSA and CSB gene-deficient cells cannot repair the DNA damage encountered during the transcription process, causing RNA polymerase to stagnate at this location, interfering with gene expression, leading to cell dysfunction or cell death, and ultimately to CS. At present, 97 kinds of CSB gene mutations have been reported to be associated with Cockayne syndrome. In addition to CSB pure mutations, CSB heterozygous mutations can also cause CS. Among them, the structure of CSB protein and the types of pathogenic mutations are shown in Figure 1 [2].
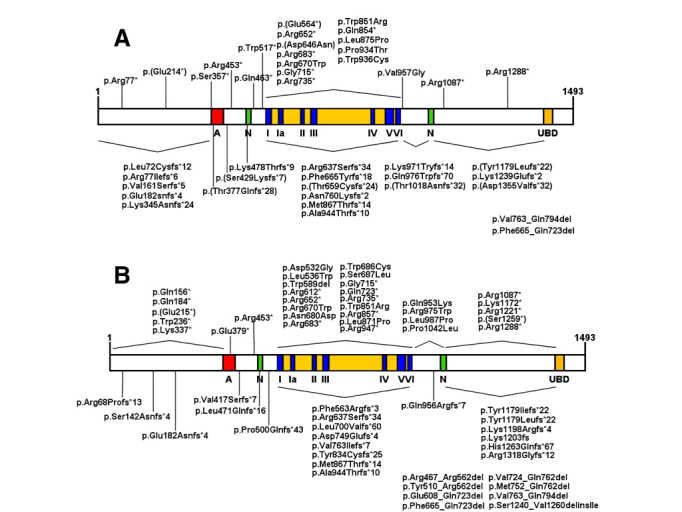
Figure 1. Representation of CSB protein and domains [2] . Acidic region (A), nuclear localization signal (N), helicase motifs (I, Ia -VI) and ubiquitin binding domain (UBD). (A) Homozygous mutations are indicated: frameshifts and nonsense mutations are indicated above the protein, while deletions and missense mutations are indicated below the protein. (B) Represents heterozygous mutations.
CS Related Mouse Model for Research
Gorgels et al. established a CS mouse model of nucleoside excision repair disorder by imitating the truncation of the CSB (ERCC6) gene in human CS patients. The results showed that CSB gene-specific truncated mice (Csb -/- ) have some characteristics similar to human CS, such as defects in transcription coupled repair, hypersensitivity to ultraviolet light, spontaneous retinal degeneration, and the inability to restore RNA synthesis after ultraviolet exposure. But compared with CS patients, Csb -/- mice are more susceptible to skin cancer, especially squamous cell carcinoma. This is because humans mainly deal with ultraviolet-induced cyclobutane pyrimidine dimers through the genome-wide repair (GG-NER) pathway, while mice preferentially repair ultraviolet-induced cyclobutane pyrimidine dimers through the TC-NER pathway. The GG-NER function of CS patients and Csb -/- mice is not affected. Therefore, Csb -/- mice cannot process the ultraviolet-induced cyclobutane pyrimidine dimer due to TC-NER deficiency, resulting in Csb -/- The susceptibility of mice to cancer is increased [3].
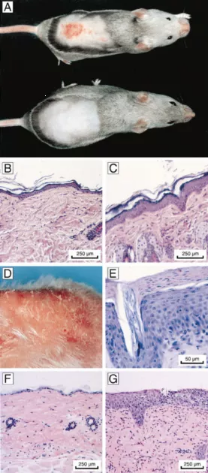
Figure 2. Acute and Chronic Effects in the Skin of CSB-Deficient Mice after Exposure to UV or DMBA [3]
(A) UV-induced erythema in the skin of CSB -/- mice. Shaven animals were exposed to UV-B (1000 J/m2 /day) for 4 consecutive days (photographs taken 1 week after the first exposure). (Top) CSB -/- male mouse (note reduced size). (Bottom) Wild-type male mouse.
(B and C) Skin sections of shaven CSB +/+ (B) and CSB -/- (C) mice after exposure to UV-B (200 J/m2 /day) for 4 consecutive days. The wild-type skin appears normal. The CSB-deficient skin shows hyper-plasia (acanthosis and hyperkeratosis).
(D and E) Skin of a CSB -/- mouse after chronic exposure to UV-B (cumulative dose 30 kJ/m2). Macroscopic changes include redness and scaling (D), and histologicchanges include acanthosis and para-keratosis (E).
(F and G) Skin sections of shaven CSB +/+ (F) and CSB -/- (G) mice after three weekly treatments with 5 μg DMBA. The wild-type skin shows normal morphology. The CSB-deficient skin demonstrates strong hyperplasia (acanthosis).
Treatment of Cockayne Syndrome
Regarding CS, symptomatic treatment is currently mainly adopted, such as physical therapy and minor operations on the affected organs, such as removal of cataracts. Those who are very sensitive to UV radiation are advised to wear UV protective clothing.
In response to progeria, the Third Hospital of Beijing Medical University used stem cell therapy to treat a 14-year-old girl with progeria, making it the first international case of placental stem cells to treat progeria. The Third Hospital of Beijing Medical University gave birth to a normal baby boy using test-tube baby technology. Subsequently, mesenchymal stem cells (MSC) were isolated from the baby boy’s placenta and transplanted into the sick sister. Clinical testing found that the patient's liver function and other indicators have improved, and the symptoms of the disease have alleviated.
In addition, Wang et al. reprogrammed fibroblasts from CS patients with ERCC6 mutations into induced pluripotent stem cells (iPSCs), and used the CRISPR/Cas9 system to further generate genetically corrected CS-iPSCs (GC-iPSCs). CS-related phenotypic defects also appeared in mesenchymal stem cells (CS-MSCs) and neural stem cells (CS-NSCs) induced and differentiated by CS-iPSCs, and these two types of cells are more susceptible to DNA damage stress. However, targeted correction of ERCC6 mutant genes can improve the premature aging defect of CS-MSCs. Therefore, the use of CRISPR/Cas9 to target and correct the pathogenic gene mutations of autologous iPSCs, and then to induce differentiation into GC-MSCs without pathogenic mutations, will be a feasible individualized treatment plan for CS, which will open up stem cell therapy to treat CS. A new idea [4] .
Summary
ERCC6 is the main pathogenic gene of CS. Mutations in the CSB gene will cause cells to be unable to repair the DNA damage encountered during the transcription process, thereby causing RNA polymerase to stagnate at this position, interfering with gene expression, and ultimately causing CS disease. At present, the clinical treatment methods applied to CS can only relieve the symptoms of CS, but cannot cure the disease. Although the first case of using a brother’s healthy MSC transplantation to treat CS has been reported, the safety of long-term use of stem cells for allogeneic therapy remains to be seen. In addition to allogeneic MSC therapy, studies have also shown that CS-iPSCs are produced through somatic cell reprogramming technology, and then targeted correction of pathogenic gene mutations are achieved through CRISPR/Cas9, and then targeted differentiation into autologous GC-MSCs can improve CS-MSCs. However, this method has not yet undergone clinical trials, and strict safety assessments are required before application.
References:
[1] Li Dongxiao, Yang Yanling. Clinical and genetic research progress of Cockayne syndrome[J]. Chinese Journal of Practical Pediatrics, 2018,33 (9): 714-717. DOI: 10.3760/cma.j.issn.2095- 428X.2018.09.015.
[2] Vessoni AT, Guerra CCC, Kajitani GS, Nascimento LLS, Garcia CCM. Cockayne Syndrome: The many challenges and approaches to understand a multifaceted disease [J]. Genet Mol Biol. 2020 May 20;43(1 suppl. 1) :e20190085. doi: 10.1590/1678-4685-GMB-2019-0085.
[3] van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, Morreau H, Beems RB, van Kreijl CF, de Gruijl FR, Bootsma D, Hoeijmakers JH. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition [J]. Cell. 1997 May 2;89(3):425-35. doi: 10.1016/s0092-8674(00)80223-8.
[4] Wang S, Min Z, Ji Q, Geng L, Su Y, Liu Z, Hu H, Wang L, Zhang W, Suzuiki K, Huang Y, Zhang P, Tang TS, Qu J, Yu Y, Liu GH , Qiao J. Rescue of premature aging defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction [J]. Protein Cell. 2020 Jan;11(1):1-22. doi: 10.1007/s13238-019-0623 -2.




영업일 기준 1-2일 내에 답변해 드리겠습니다.